Sowing Tiny Seeds on Media
ASOP-0008 Version 1
- Last Revision:
Table of Contents
1.0: Purpose
The purpose of this SOP is to provide instructions on how to sow seeds of Arabidopsis thaliana (or other ‘tiny’ plant seeds) onto media (typically space media) for astrobotany experiments, with an emphasis on reducing contamination.

2.0: Safety
Standard laboratory personal protective equipment (PPE) should be worn when executing this technique. Refer to Material Safety Data Sheets (SDS) for hazards and appropriate handling precautions. 70% ethanol is flammable and should be handled and stored appropriately.
3.0: Definitions and Acronyms
- ASOP: Astrobotany Standard Operating Procedure
- A. thaliana: Shorthand for Arabidopsis thaliana
- BSC: Biosafety Cabinet/Fume Hood
- EtOH: Shorthand for Ethanol
- NLT: No Less Than
4.0: Materials
Equivalent Materials may be used.
Note: It is recommended to use “sterile” materials if possible. Any materials can be used, but new materials that are opened directly inside the fume hood/biosafety cabinet will significantly reduce risk of contamination.
- 70% Ethanol
- 70% Ethanol Wipes or Paper Towels
- Toothpicks
- Filter Paper
- Micropore Tape
- 90 mm square Petri dishes w/ Space Media (prepared per ASOP-0003: Making Space Media)
5.0: Equipment
Equivalent Equipment may be used.
Note: It is recommended to perform this preparation in a clean BSC to reduce risk of contamination, however, if BSC is unavailable- this preparation can be performed outside of a BSC as well.
- BSC (Biosafety Cabinet/Fume Hood)
6.0: Procedure
Note: If a BSC is unavailable, skip to step 5 to start.
- Wipe down the BSC interior with 70% EtOH and let the blower run for NLT 20 minutes.
- Put gloves on and the wipe down laboratory gloves with 70% EtOH and briefly let dry.
- Wipe down the outside of bulk materials with 70% EtOH if possible before placing in the fume hood (i.e. if prepared plates are in a plastic sleeve, wipe down the outside only.)
- Designate half of the BSC workspace as ‘Zone 1’ and the other half as ‘Zone 2’ (see Fig 6.1). Bring all of the ‘bulk’ materials into Zone 2- this is where they will remain as seeds are sown in Zone 1. Try to keep the workflow as unidirectional as possible- materials from the outside should come into Zone 2 -> Seeds are then sowed on plates in Zone 1 -> then the finished plates are removed from Zone 1 to the outside. Avoid moving materials from Zone 1 to Zone 2 to reduce risk of contamination.
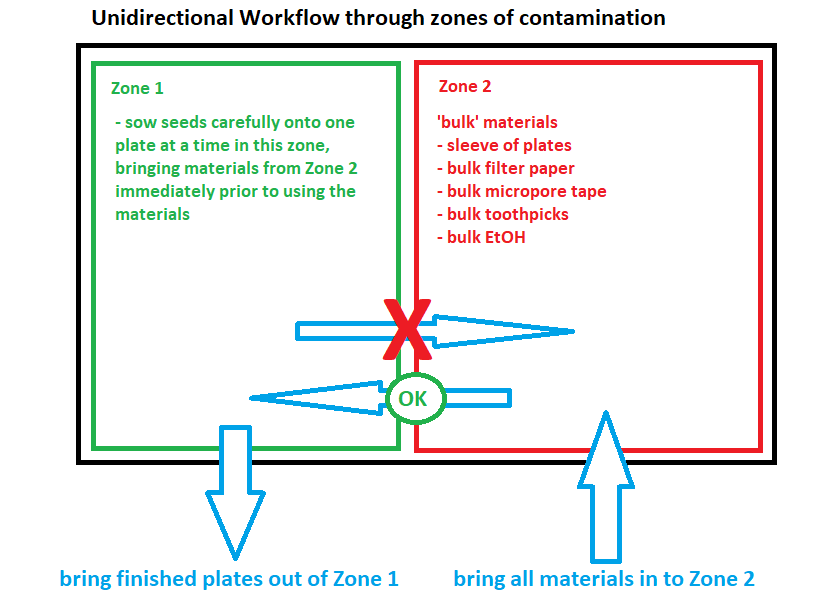
Fig 6.1: Unidirectional Workflow through Zones of Contamination - Soak a piece of filter paper with EtOH and dump the Arabidopsis thaliana seeds onto it.
- Dip a toothpick in EtOH and carefully use the end to pick up a single Arabidopsis seed. This technique takes practice, but make sure the end of the toothpick is wet so it is able to pick up a small seed.
- Open a prepared petri dish with space media and sow the seeds as desired by gently placing the seed on the surface of the gel agar. Placing 6 seeds in a straight row is good practice (to give enough distance between seeds)- but up to 10 can be placed in a single row on the dish, if necessary.
- Place the lid back on the petri dish after seeds are sowed on the media. Take the micropore tape and wrap area where the lid meets the plate 2-3 times.
7.0: References
- ASOP-0001: Ethanol Seed Sterilization
- ASOP-0003: Making Space Media
Version History
- Version 1 | Description: ASOP-0008 page created. | Effective: 01Aug2023
